Dow University of Health Sciences (DUHS OJHA)
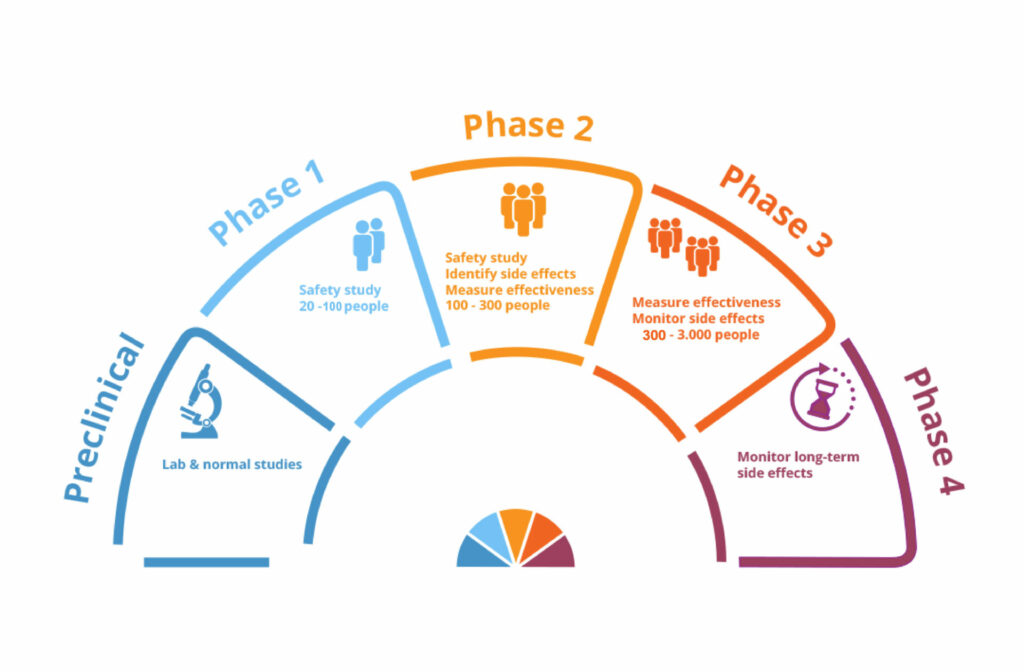
Dow University of Health Sciences Ojha Campus is approved by the Drug Regulatory Authority of Pakistan as a Clinical Trial site for the Conduct of Phase-1 to Phase-4 clinical trials.

Sections
Dow University Hospital has following distinctive Sections:
- General Medicine.
- OJHA Institute Chest diseases.
- ICU and OT complex.
- National Institute of Liver &GI Disease.
- Diabetes & Endocrinology.
- General Surgery.
- Orthopedic.
- Cardiology & Cardiac Surgery.
- Urology & Renal Transplant.
- Oncology.
- Neurosurgery.
- Clinical Hematology & Bone Marrow Transplantation.
- Liver Transplant.
- Head & Neck Surgery.
- Dow Institute of Radiology.
Amenities
Clinical Trial Site office is designed with the following amenities:
| # | Amenities |
|---|---|
| 1 | Induction Hall |
| 2 | Screening Room |
| 3 | Informed Consent Room |
| 4 | Doctors Room |
| 5 | Emergency Management Room |
| 6 | Drug Administration Area |
| 7 | Accessed controlled Pharmacy |
| 8 | Sample Collection Area |
| 9 | Sample Processing & Storage Room |
| 10 | Cold Storage ranging from 02°C to -70°C |
| 11 | Observation Wards |
Sindh Infectious Disease Hospital & Research Center (SIDH) Clinical Trial Site
Sindh Infectious Disease Hospital and Research Centre (SIDH&RC) is the only Infectious Disease hospital in Sindh that has outstanding experts and dedicated facilities for the treatment of infectious diseases. SIDH&RC is also approved by the Drug Regulatory Authority of Pakistan for the conduct of Phase-1 to Phase-4 clinical studies.

Facilities
The facilities available at SIDH&RC are:
- Sindh Infectious Diseases Hospital & Research Centre (SIDH & RC) is a 175-bedded hospital.
- It houses adult units with 48 ICU beds and 68 HDU beds, along with paediatric units having 8 PICU beds and 34 HDU beds.
- The hospital maintains twelve isolation rooms with negative pressure to implement airborne precautions, ensuring the prevention of contagious diseases.
- Challenging cases such as HIV/AIDS, Diphtheria, Tuberculosis, and Crimean Congo Haemorrhagic Fever (CCHF) can also be managed.
- Well-equipped wards to manage infectious diseases, pulmonology, nephrology, neurology, and paediatric infectious diseases.
Amenities
Clinical Trial Site office is designed with the following amenities:
| # | Amenities |
|---|---|
| 1 | Induction Hall |
| 2 | Screening Room |
| 3 | Informed Consent Room |
| 4 | Doctors Room |
| 5 | Emergency Management Room |
| 6 | Drug Administration Area |
| 7 | Accessed controlled Pharmacy |
| 8 | Sample Collection Area |
| 9 | Sample Processing & Storage Room |
| 10 | Cold Storage ranging from 02°C to -70°C |
| 11 | Observation Wards |
Clinical Study Process
To request a study, please download and fill the form by clicking the button and email it to bd.ibbps@duhs.edu.pk
Contact Us
INSTITUTE OF BIOLOGICAL, BIOCHEMICAL & PHARMACEUTICAL SCIENCES (IBBPS)
